Hymenomycetes
Jelly Fungi, Yeasts, and Mushrooms
David S. Hibbett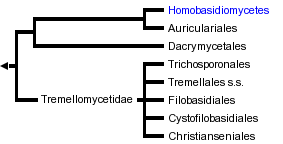

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The Hymenomycetes is one of three major clades of Basidiomycota (the others are the Urediniomycetes and Ustilaginomycetes). The Hymenomycetes contains roughly 20,000 described species, which is almost 70% of the (known) Basidiomycota. About 98% of the species of the Hymenomycetes are in a clade called the Homobasidiomycetes, which includes mushrooms, bracket fungi, puffballs, and others. The remaining clades are the Tremellomycetidae, Dacrymycetales, and Auriculariales. These latter groups include "jelly fungi" (Fig. 1), which have gelatinous, often translucent fruiting bodies (e.g., "witches butter" Tremella mesenterica), as well as many yeast-forming species.
 image info
image info
Figure 1. Phlogiotis (Tremiscus) helvelloides (Auriculariales).
? David S. Hibbett.
Hymenomycetes display the full range of ecological strategies that characterizes the Basidiomycota as a whole. To obtain carbon nutrition, Hymenomycetes decompose dead organic matter or enter into diverse associations (both antagonistic and benign) with plants, animals, and other fungi. Mycorrhizal associations with plants are present in many lineages of Homobasidiomycetes, and have also been demonstrated in some Auriculariales (Selosse et al. 2002). Mycoparasitism (parasitism of a fungus by a fungus) is especially widespread in the Tremellomycetidae (Bandoni 1984). Few Hymenomycetes are medically important. An exception is Filobasidiella neoformans (also called Cryptococcus neoformans), which is a serious pathogen of immunocompromised individuals (Mitchell and Perfect 1995).
Hymenomycetes include the vast majority of edible mushrooms, most of which are in the Homobasidiomycetes. Several jelly fungi are cultivated for food in Asia, including the "wood ear" fungus, Auricularia auricula-judae (Fig. 2), and the white jelly fungus, Tremella fuciformis.
 image info
image info
Figure 2. Cultivated fruiting bodies of Auricularia auricula-judae (Auriculariales).
? David S. Hibbett.
Characteristics
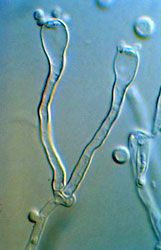
The Hymenomycetes are quite variable in both macroscopic and microscopic features. Traditionally, one of the most important characters in the higher-level taxonomy of Basidiomycota has been the form and septation of the basidia (the cells in which meiosis occurs, and on which sexual spores are formed) (Talbot, 1968; McNabb, 1973). Basidia of Hymenomycetes have various shapes and may be undivided or divided by transverse or longitudinal septa (Fig. 3). Most Hymenomycetes produce four spores on each basidium, but some species produce as few as one or as many as eight spores per basidium. Another important character in prior taxonomy of Basidiomycota is the presence or absence of "spore repetition", which is the production of secondary spores directly from basidiospores. The Hymenomycetes display variation in this character also. It is therefore not surprising that the Hymenomycetes, as delimited here, was not formally recognized as a taxonomic group before the advent of molecular characters.
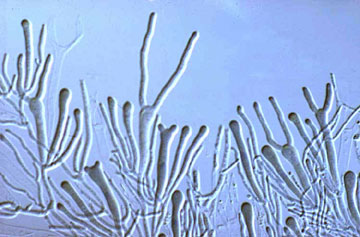 image info
image info
Figure 3. Two-spored "tuning fork" basidia of the jelly fungus Dacrymyces.
? George Barron. From George Barron's Website on Fungi used with permission.
The one unifying morphological feature of the Hymenomycetes is the presence of "dolipore" septa, which are pores in the crosswalls between adjacent cells that are bordered by a collar-like margin of cell wall material (Fig. 4). Other Basidiomycota also have septal pores, but their structure is not the same. Flanking the dolipores in almost all Hymenomycetes is a membrane-bound structure called a "septal pore cap". These structures are also called "parenthesomes", because they resemble parentheses around the septal pores when viewed in thin sections with transmission electron microscopy.
Variation in the form of the parenthesomes has provided clues to phylogenetic relationships in the Hymenomycetes (Adams et al. 1995; Bandoni and Oberwinkler 1982; Hibbett and Thorn 2001; McLaughlin et al. 1995; Moore, 1985; Swann and Taylor 1993; Wells and Bandoni 2001). Tremellomycetidae have "vesiculate" parenthesomes, in which the septal pore cap is divided into cup-shaped sections. Dacrymycetales and Auriculariales have "imperforate" parenthesomes, in which the septal pore cap forms a smooth, dome-shaped covering over the dolipore. Homobasidiomycetes contain a mixture of imperforate and "perforate" parenthesomes. In the latter, the septal pore cap has openings of varying sizes, and appears discontinuous in section. A few species in the Tremellomycetidae and Homobasidiomycetes are reported to lack parenthesomes.
 image info
image info
Figure 4. Dolipore septa with perforate parenthesome (septal pore cap).
From Moore and Marchant (1972); used with permission.
Most known species of Homobasidiomycetes are filamentous and produce a multicellular fruiting body. The fruiting body is not always what most people would regard as a "mushroom", however. For example, in "resupinate" species the fruiting body is a flattened structure, which may be little more than a thin layer of hyphae produced on the underside of a log or some other inconspicuous location. As noted, some Hymenomycetes are yeasts and produce no fruiting body at all. Fruiting bodies of Hymenomycetes reach their greatest size, diversity, and complexity in the Homobasidiomycetes, but certain jelly fungi produce elaborate fruiting bodies as well. It is likely that the yeasts and resupinate forms have been undercollected, relative to their more conspicuous relatives.
Discussion of Phylogenetic Relationships
Phylogenetic analyses of ribosomal gene (rDNA) sequences have resolved many major groups within the Hymenomycetes, but higher-level relationships among these groups are generally not resolved with confidence. The phylogenetic hypothesis and classification adopted here are based on several independent analyses of rDNA sequences (Fell, 2001; Swann and Taylor, 1993; Swann et al. 1995) and include four major clades: Tremellomycetidae, Dacrymycetales, Auriculariales, and Homobasidiomycetes (which is here taken to include the Tulasnellales and Ceratobasidiales). Some analyses suggest that the Dacrymycetales, Auriculariales, and Homobasidiomycetes form a monophyletic group, which has been called the Hymenomycetidae (Swann and Taylor 1995), but this group has not been supported by other studies (Begerow et al. 1997; Wei? and Oberwinkler 2001) and it is not adopted here.
The Dacrymycetales and Tremellomycetidae have been supported as monophyletic in several analyses (Binder and Hibbett 2002; Swann and Taylor 1993, 1995; Wei? and Oberwinkler 2001). The major problems in the phylogeny of Hymenomycetes concern the monophyly of the Auriculariales and the placements of the Tulasnellales and Ceratobasidiales. Space limitations preclude a thorough discussion of all the alternative phylogenetic hypotheses. To illustrate the current controversies, we will contrast the present classification with the classification of Kirk et al. (2001) and the results of a phylogenetic study by Wei? and Oberwinkler (2001).
Kirk et al. (2001) presented one of the major contemporary classifications for Basidiomycota in the Dictionary of the Fungi 9th edition. The classification adopted here differs from that in the Dictionary in several ways: 1) The Dictionary classification calls the Hymenomycetes the "Basidiomycetes" (not to be confused with "basidiomycetes", which is a general term for all Basidiomycota); 2) The Dictionary classification includes the Tulasnellales, Ceratobasidiales, Dacrymycetales, and Auriculariales in the Tremellomycetidae, whereas the present classification places the Tulasnellales and Ceratobasidiales (resupinate forms with septate or non-septate basidia, respectively) in the Homobasidiomycetes, and treats the Dacrymycetales and Auriculariales as outside of the Tremellomycetidae; and, 3) The Dictionary classification calls the Homobasidiomycetes the "Agaricomycetidae" (and excludes Tulasnellales and Ceratobasidiales).
Table 1. Comparison of the present classification of Hymenomycetes and that of Kirk et al. (2001).
| Present classification | Kirk et al. (2001) |
|---|---|
|
|
Wei? and Oberwinkler (2001) performed a phylogenetic analysis of nuclear large subunit (nuc-lsu) rDNA with excellent taxonomic coverage of Auriculariales, as well as exemplars of other groups of Hymenomycetes (Fig. 5). In Wei? and Oberwinkler's tree, the Auriculariales is shown to be a polyphyletic group, with clades that correspond to 1) Auriculariales s. str.; 2) Sebacinaceae; and, 3) a minor clade with two species, Ceratosebacina calospora and Exidiopsis gloeophora. Wei? and Oberwinkler's results also suggested that the Ceratobasidiales is the sister group of the Dacrymycetales. The Tulasnellales was not included in their dataset, but unpublished analyses using nuclear small subunit (nuc-ssu) rDNA sequences were cited suggesting that the Tulasnellales are closely related to the Auriculariales (Wei? and Oberwinkler 2001). The results of Wei? and Oberwinkler (2001) are consistent with the delimitation of the Agaricomycetidae by Kirk et al. (2001), which excludes Tulasnellales, Ceratobasidiales, and Sebacinaceae. Many of the key nodes that support placement of major groups in the analysis of Wei? and Oberwinkler (2001) received weak bootstrap support, however.
 image info
image info
Figure 5. Schematic representation of the phylogenetic tree of Wei? and Oberwinkler (2001). Numbers along branches indicate bootstrap frequencies. Bootstrap values below 60% are not shown.
The studies used as the basis of the classification presented here suggest that 1) the Auriculariales s. str. may be monophyletic or paraphyletic; and, 2) the Tulasnellales, Ceratobasidiales, and Sebacinaceae are part of a group of closely related taxa that are nested within the cantharelloid clade of the Homobasidiomycetes, which includes the edible chanterelle (Cantharellus cibarius) and others (Bruns et al. 1998; Hibbett and Binder 2002; Hibbett and Donoghue 2001; Langer 1998). Bruns et al. (1998) performed an analysis of mitochondrial large subunit rDNA sequences that grouped an isolate of "Sebacina sp." and Tulasnella irregularis (Tulasnellales), and placed that group as the sister group to Cantharellus, with strong (98%) bootstrap support. Hibbett and Donoghue (2001) performed an analysis that placed Tulasnella in the cantharelloid clade with 74% bootstrap support. A later analysis by Hibbett and Binder (2002) supported the monophyly of the cantharelloid clade, including Uthatobasidium and Thanatephorus (Ceratobasidiales) and Tulasnella, as well as Cantharellus and other Homobasidiomycetes (there was no bootstrap analysis in that study). Unpublished analyses of Hibbett and Binder suggest that Sebacina vermifera (as Serendipita vermifera; Sebacinaceae) is also in the cantharelloid clade.
There appears to be an elevated rate of evolution of nuclear rDNA genes in some members of the cantharelloid clade, which is a potentially serious source of error in phylogenetic studies that use these genes. Species of Cantharellus and Craterellus (both Cantharellaceae) have divergent nuc-ssu rDNA sequences that cannot be aligned in their entirety to other Homobasidiomycetes and produce conspicuously long branches in phylograms (Hibbett et al. 1997). Nuc-ssu rDNA sequences of Tulasnella are even more divergent than those of Cantharellus (Hibbett, unpublished).
References
Adams, G. C., Klomparens, K. L., Hennon, P. E. 1995. Unusual reticulated parenthesomes surround the dolipore of a hyphomycete with clamp connections, Ditangifibulae dikaryotae gen. et sp. nov. Mycologia 87:909-921.
Alexopoulos, C.J., Mims, C.W. and Blackwell, M. 1996. Introductory Mycology. John Wiley and Sons, New York.
Bandoni, R. J. 1984. The Tremellales and Auriculariales: and alternative classification. Trans. Mycol. Soc. Japan 25:489-530.
Bauer, R., Begerow, D., Oberwinkler, F., Piepenbring, M. and Berbee, M. L 2001. Ustilaginomycetes. Pp. 57-84. In: The Mycota VII. Systematics and Evolution. Part B. (Mclaughlin, D. J., McLaughlin, E. G. and Lemke, P. A., eds.). Springer-Verlag, Berlin.
Begerow, D., Bauer, R., and Oberwinkler, F. 1997. Phylogenetic studies on nuclear large subunit ribosomal DNA sequences of smut fungi and related taxa. Can. J. Bot. 75:2045-2056.
Bruns, T. D., Szaro, T. M., Gardes, M., Cullings, K.W., Pan, J. J., Taylor, D. L., Horton, T. R., Kretzer, A., Garbelotto, M., Li, Y. 1998. A sequence database for the identification of ectomycorrhizal basidiomycetes by phylogenetic analysis. Molec Ecol 7:257-272.
Chen, C.-J. 1998. Morphological and molecular studies in the genus Tremella. Bibliotheca Mycologica 174:1-225.
Fell, J. W., Boekhout, T., Fonseca, A. and Sampaio J.P. 2001. Basidiomycetous yeasts. Pp. 1-36. In: The Mycota VII. Systematics and Evolution. Part B. (Mclaughlin, D. J., McLaughlin, E. G. and Lemke, P. A., eds.). Springer-Verlag, Berlin.
Hibbett, D. S. and Binder, M. 2002. Evolution of complex fruiting-body morphologies in homobasidiomycetes. Proc. R. Soc. Lond. B 269:1963-1969.
Hibbett, D. S. and Donoghue, M. J. 2001. Analysis of character correlations among wood decay mechanism, mating systems, and substrate ranges in homobasidiomycetes. Syst. Biol. 50:215-242.
Hibbett, D. S., Pine, E. M., Langer, E., Langer, G. and Donoghue, M. J. 1997. Evolution of gilled mushrooms and puffballs inferred from ribosomal DNA sequences. Proc. Nat. Acad. Sci. USA 94:12002-12006.
Hibbett, D. S. and Thorn, R. G. 2001. Homobasidiomycetes. Pp. 121-170. In: The Mycota VII. Systematics and Evolution. Part B. (Mclaughlin, D. J., McLaughlin, E. G. and Lemke, P. A., eds.). Springer-Verlag, Berlin.
Ingold, C. T. 1985. Observations on spores and their germination in certain heterobasidiomycetes. Trans. Br. Mycol. Soc. 85:417-423.
Jülich, W. 1981. Higher taxa of basidiomycetes. J. Cramer, Leichtenstein.
Kirk, P.M., Cannon, P.F., David, J.C., and Stalpers, J. 2001. Ainsworth and Bisby’s Dictionary of the Fungi. 9th ed. CAB International, Wallingford, UK.
Langer E. 1998. Evolution of Hyphodontia (Corticiaceae, Basidiomycetes) and related Aphyllophorales inferred from ribosomal DNA sequences. Folia Cryptog. Estonica Fasc. 33:57-62.
McLaughlin, D.J., Frieders, E.M. and Lü, Haisheng. 1995. A microscopist's view of heterobasidiomycete phylogeny. Stud. Mycol. 38: 91-109.
McNabb, R. F. R. 1973. Phragmobasidiomycetidae: Tremellales, Auriculariales, Septobasidiales. Pp. 303-316 in G. C. Ainsworth, F. K. Sparrow and A. S. Sussman (eds.) The Fungi. An advanced Treatise. Vol IVB. Academic press, New York.
McNabb, R. F. R., and Talbot, P. H. B. 1973. Holobasidiomycetidae: Exobasidiales, Brachybasidiales, Dacrymycetales, Tulasnellales. Pp. 317-326 in G. C. Ainsworth, F. K. Sparrow and A. S. Sussman (eds.) The Fungi. An advanced Treatise. Vol IVB. Academic press, New York.
Mitchell T. G. and Perfect, J. R. 1995. Cryptococcosis in the era of AIDS – 100 years after the discovery of Cryptococcus neoformans. J. Clin. Microbiol. Rev. 8:515-548.
Moore, R. T. 1985. The challenge of the dolipore/parenthesome septum. Pp. 175-212, in: D. Moore, L. A. Casselton, D. A. Wood, and J. Frankland (eds.) Developmental biology of higher fungi. Cambridge University Press, Cambridge.
Moore, R. T. and Marchant, R. 1972. Ultrastructural characterization of the basidiomycete septum of Polyporus biennis. Can. J. Bot. 50:2463-2469.
Müller, W. H., Stalpers, J. A., van Aelst, A. C., van der Krift, T. P., and Boekhout, T. 1998. Field emission gun-scanning electron microscopy of septal pore caps of sected species in the Rhizoctonia s.l. complex. Mycologia 90:170-179.
Oberwinkler, F. 1985 Anmerkungen zur Evolution und Systematik der basidiomyceten. Bot. Jahrb. Syst. 107, 541-580.
Oberwinkler, F. and Bandoni, R. J. 1982. A taxonomic survey of the gasteroid, auricularioid Heterobasidiomycetes. Can. J. Bot. 60:1762-1750.
Parmasto, E. 1986 On the origin of hymenomycetes (what are corticioid fungi?). Windahlia 16, 3-19.
Prillinger, H. Dörfler, C. Laaser, G. and Hauska, G. 1990. A contribution to the systematics and evolution of higher fungi: yeast-types in the basidiomycetes. Part III: Ustilago-type. Z. Mycol. 56: 251-278.
Prillinger, H., Laaser, G., Dörfler, C. and Ziegler, K. 1991. A contribution to the systematics and evolution of higher fungi: yeast-types in the basidiomycetes. Part IV: Dacrymyces-type, Tremella-type. Sydowia 43: 170-218.
Selosse, M.A., Bauer. R. and Moyersoen, B. 2002. Basal hymenomycetes belonging to Sebacinaceae are ectomycorrhizal on temperate deciduous trees. New Phytol. 155:183-195.
Smith, S. E. and Read, D. J. 1997. Mycorrhizal symbiosis. Academic Press, San Diego.
Swann, E.C. and Taylor, J.W. 1993. Higher taxa of basidiomycetes: an 18S rRNA gene perspective. Mycologia 85: 923-936.
Swann, E.C. and Taylor, J.W. 1995. Phylogenetic perspectives on basidiomycete systematics: evidence from the 18S rRNA gene. Canad. J. Bot. 73: S862-S868.
Talbot, P. H. B. 1968. Fossilized pre-Patouillardian taxonomy? Taxon 17:620-628.
Warcup, J. H. 1988. Mycorrhizal associations of isolates of Sebacina vermifera. New Phytol. 110:227-231.
Webster, J., Davey, R.A., Duller, G.A. and Ingold, C.T. 1984b. Ballistospore discharge in Itersonilia perplexans. Trans. Br. Mycol. Soc. 82: 13-29.
Weiß, M. and Oberwinkler, F. O. 2001. Phylogenetic relationships in Auriculariales and related groups-hypotheses derived from nuclear ribosomal DNA sequences. Mycol. Res. 105:403-415.
Wells, K. 1994. Jelly fungi, then and now! Mycologia 86:18-48.
Wells, K., and Bandoni, R. J. 2001. Heterobasidiomycetes. Pp. 85-120. In: The Mycota VII. Systematics and Evolution. Part B. (Mclaughlin, D. J., McLaughlin, E. G. and Lemke, P. A., eds.). Springer-Verlag, Berlin.
Information on the Internet
- Mycological Society of America. Extensive links to sites concerned with Basidiomycota and other fungi.
- The WWW Virtual Library: mycology.
- Deep Hypha Research Coordination Network. Deep Hypha is a project to coordinate and provide resources for research in fungal systematics.
- AFTOL: Assembling the Fungal Tree of Life. Collaborative research in fungal phylogenetics.
- Tom Volk's Fungi. An amazing collection of information, images, and lore about fungi.
- Australian National Botanic Gardens Fungi Web Site. Jelly fungi pages.
- Fungi of California Jelly Fungi page.
- Doctor Fungus Cryptococcus neoformans (Filobasidiella neoformans) page.
- MykoWeb. Specializing in the fungi of California.
- George Barron's Website on Fungi. Diverse fungal images by a distinguished mycologist.
- Duke University Mycology Research Unit. Information and resources about the medically important hymenomycete, Filobasidiella (Cryptococcus) neoformans.
Title Illustrations
| Scientific Name | Calocera viscosa |
|---|---|
| Comments | Dacrymycetales |
| Acknowledgements | Image from MykoWeb; used with permission |
| Copyright | © Mike Wood |
| Scientific Name | Filobasidiella (Cryptococcus) neoformans |
|---|---|
| Comments | Tremellomycetidae |
| Acknowledgements | Image from the Duke University Mycology Research Unit; used with permission |
| Copyright | © Wiley Schell |
| Scientific Name | Gomphus |
|---|---|
| Comments | Hymenomycetes |
| Copyright | © 2003 David S. Hibbett |
About This Page
Development of this page was facilitated by the "Deep Hypha" Research Coordination Network and the "Assembling the Fungal Tree of Life" project (NSF awards DEB-0090301 and DEB-0228657). George Barron, Mike Wood, Wiley Schell, and the NRC Research Press are thanked for permission to reproduce the images in Figs. 3 and 4 and the title illustration.
David S. Hibbett
Biology Department
Clark University
950 Main Street
Worcester, MA 01610
USA
Correspondence regarding this page should be directed to David S. Hibbett at
Page copyright © 2003 David S. Hibbett
- First online 22 September 2003
- Content changed 22 September 2003
Citing this page:
Hibbett, David S. 2003. Hymenomycetes. Jelly Fungi, Yeasts, and Mushrooms. Version 22 September 2003 (tol-reviewed). http://tolweb.org/Hymenomycetes/20531/2003.09.22 in The Tree of Life Web Project, http://tolweb.org/