Cladrastis clade
Martin F. Wojciechowski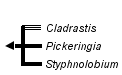

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Tribe Sophoreae has been considered a "tribe of convenience" to include the heterogenous assemblage of genera within Papilionoideae that have relatively simple flowers and unspecialized pinnate leaves (Polhill, 1994). Since Polhill's last (1994) treatment of Sophoreae, cladistic analyses of morphology (Chappill, 1995), pollen data (Ferguson et al., 1994), and molecular sequences (e.g. Doyle et al., 1997; Kajita et al., 2001; Pennington et al., 2001; Wojciechowski et al., 2004) have clearly shown Sophoreae to be paraphyletic, its constituent genera distributed as members of disparate, early-branching papilionoid lineages.
One of the more interesting clades to emerge from these analyses is the "Cladrastis" clade (Wojciechowski et al., 2004) containing the genera Cladrastis Raf., Pickeringia Nutt. ex Torrey & A. Gray, and Styphnolobium Schott. Cladrastis and Styphnolobium (segregate of Sophora L.) have been traditionally classified in Sophoreae sens. str. (Polhill, 1981, 1994), whereas Pickeringia has been classified in tribe Thermopsideae (Turner, 1981). The close relationship of Cladrastis and Styphnolobium is notable biogeographically because both genera exhibit East Asian-North American disjunctions.
Cladrastis, with 6 to 7 species of trees found in warm temperate montane evergreen, mixed-deciduous forests, is distributed in east Asia (6 species; China to Japan) and eastern North America (1 sp.; southern Illinois to North Carolina) (Duley and Vincent, 2003; Pennington et al., 2005). Styphnolobium, with 9 species of trees inhabiting temperate to seasonally-dry tropical woodlands to montane forests, is distributed from North and Central America (8 species) to west-central China (1 species) (Pennington et al., 2005). Pickeringia is a monotypic genus of spiny, xerophytic shrub restricted to the sclerophyllous chaparral and mixed evergreen-woodland forest vegetation of California and northern Baja California of Western North America (Raven and Axelrod, 1995).
Consistent with evidence of both Cladrastis (subgenus Cladrastis and subgenus Platycarpus) and Styphnolobium represented in the fossil record from the Middle Eocene (minimum 40 Ma) of Tennessee (Herendeen, 1992), age estimates based on rates analyses of molecular sequences suggest this is one of the oldest clades of papilionoids (Lavin et al., 2005).
Discussion of Phylogenetic Relationships
The "Cladrastis" clade is one of the well-supported, early branching clades of papilionoids to emerge from recent molecular analyses (Wojciechowski et al., 2004). In this clade, the genera Styphnolobium and Pickeringia are nested within a paraphyletic Cladrastis. The close relationship between these three genera is supported by similarities in floral morphology, chromosome number, and absence of quinolizidine alkaloids.
The position of Pickeringia in the Cladrastis clade is consistent with Polhill's (1981) and Sousa and Rudd's (1993) predictions of a close relationship between these three genera based on floral morphology, cytogenetic evidence, and the absence of quinolizidine alkaloids in Pickeringia of the type that is characteristic of other members of Thermopsideae (Turner, 1981). Recent analyses have failed to detect similar alkaloids in Cladrastis and Styphnolobium (Kite and Pennington, 2003), a finding consistent with the molecular data showing a close phylogenetic relationship between these three genera.
References
Chappill, J. A. 1995. Cladistic analysis of the Leguminosae: development of an explicit hypothesis. Pages 1-9 in Advances in Legume Systematics, part 7, phylogeny (W.D. Crisp and J.J. Doyle, eds.). Royal Botanic Gardens, Kew, UK.
Doyle, J. J., J. L. Balinger, E. E. Dickson, T. Kajita, and H. Ohashi. 1997. A phylogeny of the chloroplast gene rbcL in the Leguminosae: taxonomic correlations and insights into the evolution of nodulation. American J. Botany 84: 541-554.
Duley, M. L., and M. A. Vincent. 2003. A synopsis of the genus Cladrastis (Leguminosae). Rhodora 105: 205-239.
Ferguson, I. K., B. D. Schrire, and R. Shepperton. 1994. Pollen morphology of the tribe Sophoreae and relationships between subfamilies Caesalpinioideae and Papilionoideae. Pages 53-96 in Advances in Legume Systematics, part 6, structural botany (I. K. Ferguson and S. C. Tucker, eds.). Royal Botanic Gardens, Kew, UK.
Herendeen, P. S. 1992. The fossil history of Leguminosae from the Eocene of southeastern North America. Pages 85-160 in Advances in Legume Systematics, part 4, the fossil record (Herendeen, P.S., and D.L. Dilcher, eds.). Royal Botanic Gardens, Kew, UK.
Kajita, T., H. Ohashi, Y. Tateishi, C. D. Bailey, and J. J. Doyle. 2001. rbcL and legume phylogeny, with particular reference to Phaseoleae, Millettieae, and Allies. Systematic Botany 26: 515-536.
Kite, G. C., and R. T. Pennington. 2003. Quinolizidine alkaloid status of Styphnolobium and Cladrastis (Leguminosae). Biochemical Systematics and Ecology 31: 1409-1416.
Lavin, M., P. S. Herendeen, and M. F. Wojciechowski. 2005. Evolutionary rates analysis of Leguminosae implicates a rapid diversification of lineages during the Tertiary. Syst. Biol. 54: 530-549.
Pennington, R. T., M. Lavin, H. Ireland, B. Klitgaard, J. Preston, and J.-M. Hu. 2001. Phylogenetic relationships of basal papilionoid legumes based upon sequence of the chloroplast trnL intron. Systematic Botany 26: 537-556.
Pennington, R. T., C. H. Stirton, and B. D. Schrire. 2005. Sophoreae. Pages 227-249 in Legumes of the World (Lewis et al., eds.). Royal Botanic Gardens, Kew, UK.
Polhill, R. M. 1981. Sophoreae. Pages 213-230 in Advances in legume systematics, part 1 (R. M. Polhill and P. H. Raven, eds.). Royal Botanic Gardens, Kew, UK.
Polhill, R. M. 1994. Classification of the Leguminosae. Pages xxxv – xlviii in Phytochemical dictionary of the Leguminosae (F. A. Bisby, J. Buckingham, and J. B. Harborne, eds.). Chapman and Hall, New York, NY.
Raven, R. H., and D. I. Axelrod. 1995. Origin and relationships of the California flora. California Native Plant Society, Sacramento, California, USA.
Sousa, M., and V. E. Rudd. 1993. Revision del genero Styphnolobium (Leguminosae: Papilionoideae: Sophoreae). Annals of the Missouri Botanical Garden 80: 270-283.
Turner, B. L. 1981. Thermopsideae. Pages 403-407 in Advances in Legume Systematics, part 1 (R. M. Polhill and P. H. Raven, eds.). Royal Botanic Gardens, Kew, UK.
Wojciechowski, M. F., M. Lavin, and M. J. Sanderson. 2004. A phylogeny of legumes (Leguminosae) based on analysis of the plastid matK gene resolves many well-supported subclades within the family. Am. J. Bot. 91: 1846-1862.
Title Illustrations

| Scientific Name | Cladrastis lutea (F. Michx.) K. Koch |
|---|---|
| Location | Salisbury University Arboretum |
| Specimen Condition | Live Specimen |
| Copyright | © 2008 Salisbury University Arboretum |
| Scientific Name | Styphnolobium japonicum (L.) Schott |
|---|---|
| Comments | Commonly called Japanese Pagodatree or Scholar-tree. |
| Specimen Condition | Live Specimen |
| Copyright | © Mark Brand |
| Scientific Name | Pickeringia montana var. tomentosa |
|---|---|
| Comments | Commonly called Chaparral Pea. |
| Specimen Condition | Live Specimen |
| Source Collection | CalPhotos |
| Copyright | © 1996 |
About This Page
Martin F. Wojciechowski

Arizona State University, Tempe, Arizona, USA
Correspondence regarding this page should be directed to Martin F. Wojciechowski at
Page copyright © 2006 Martin F. Wojciechowski
All Rights Reserved.
- First online 14 June 2006
- Content changed 14 June 2006
Citing this page:
Wojciechowski, Martin F. 2006. Cladrastis clade. Version 14 June 2006 (under construction). http://tolweb.org/Cladrastis_clade/60329/2006.06.14 in The Tree of Life Web Project, http://tolweb.org/









 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site